Thursday, December 15, 2011
Trends that Affect Boiling Point (ORGANIC)
Molecular Forces of Attraction:
http://library.thinkquest.org/C006669/data/Chem/bonding/inter.html
Monday, December 12, 2011
Sound Waves
- sound waves CANNOT travel through a vacuum (limited to vibrations in a medium)
- Rarefaction: a decrease in the density of something; "a sound wave causes periodic rarefactions in its medium" (6 BR2)
- "compressions & rarefactions"
- Adiabatic: any process that occurs WITHOUT heat transfer (ex: engine breaking) ( ΔT ≠ 0 but Q = 0)
- Isothermal Process: any process that occurs WITHOUT a change in temperature (ΔT = 0 but Q ≠ 0)
- the speed of sound in air at room temp. is about 340m/s!
- vsound in solid > vsound in liquid > vsound in gas
- because solids have stronger forces between them (IMFs), thus greatest restoring force, thus can participate in another compression wave faster, thus can propogate SOUND faster in the medium (6 BR2)
β = 10 log10 (I/I0)
- Intensity (W/m2) – very LARGE number based on factors of 10, needs to be paired with a LOG expression
- Decible (β) – smaller, more reasonable number, because the answer to a log-based expression!
*** for every drop in intensity by a factor of 10, the decibel level decreases by 10:
- ex: if the intensity of a source increases by 1000 (3 factors of 10), then the decibel level of that sound will increase by 30 (3 x 10)
- ex2: if the intensity (I) increases by a factor of 10, then the intensity level (dB) increases by +10 dB. If the intensity increases by a factor of 100, then the intensity level increases by +20 dB. (USE THIS FOR ANSWER APPROXIMATION!!! Will probably be too complicated/time-wasting to sit and calculate actual logarithms!)
Intensity & Distance: the intensity of sound dissipates with distance by a SQUARED factor
ex: (Kaplan 371) Increasing distance by a factor of 10 decreases intensity by a factor of 100, therefore reducing the sound level by -20 dB.
Thursday, December 1, 2011
Sunday, November 27, 2011
Tyndall Effect (particles interacting with light)
Tyndall Effect: The scattering of light by colloidal-size particles (NOT particles in a "true solution"--those are too small). Beam of light ("path of the light") is visible/detectable.
COLLOIDAL PARTICLES ARE BIG ENOUGH TO SCATTER THE LIGHT.
http://www.youtube.com/watch?v=k5HMVIb4J7A&feature=related
 BLUE SKY / RED SUNSET
BLUE SKY / RED SUNSEThttp://www.youtube.com/watch?v=Eo1WoKfJfkA&feature=related
- blue light scatters MORE EASILY than red light
- red/orange light scatters the LEAST
- gradual addition of colloidal particles makes it gradually more difficult for the light to pass through "the container"--"as it gets saturated...more and more light gets scattered at the beginning...will see the differentiation of colors of red, orange, and blue (sunset)"
- observing light traveling the LONGEST/FURTHEST throughout the atmosphere (orange/red) as opposed to traveling DIRECTLY/SHORTEST throughout the atmosphere (blue)
http://www.youtube.com/watch?v=sEB-6uxtxyE&feature=related

Wednesday, October 5, 2011
Vital Capacity




VC = IRV + TV + ERV (nooooot RV--residual volume--too)
(703+)
- Boyle's Law! (P and V inverse)
spirometer - measures the volume of air exchanged in breathing
- normal = tidal volume (TV) = 500mL
- extra expiration = expiratory reserve volume (ERV)
- extra inspiration = inspiratory reserve volume (IRV)
- amount of air that cannot be forcibly expired = residual volume (RV)
- in pneumothorax, the RV is eliminated when lung collapses!
Thursday, July 14, 2011
Converging & Diverging Lenses
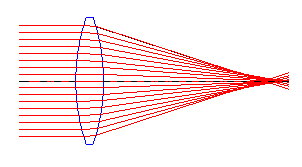 | 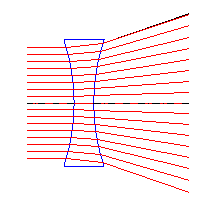 |
| Converging lens | Diverging lens |
Buffer Solutions
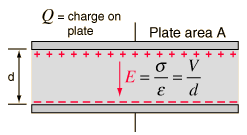
Capacitor
Wednesday, July 13, 2011
Newton's Laws
- First law: The velocity of a body remains constant unless the body is acted upon by an external force.[3][4][5]
- Second law: The acceleration a of a body is parallel and directly proportional to the net force F and inversely proportional to the mass m, i.e.,F = ma.
- Third law: The mutual forces of action and reaction between two bodies are equal, opposite and collinear.
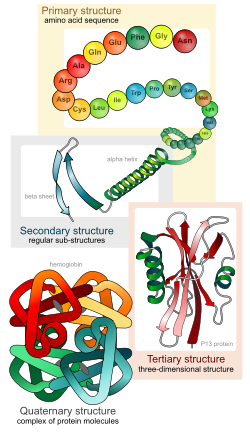
Amino Acids & Proteins
Spermatogenesis & Oogenesis (production of gametes)


Tuesday, July 12, 2011
Phase Diagram, Vapor-Liquid Equilibrium



Kaplan Pains In My Ass
Positive and negative azeotropes
Each azeotrope has a characteristic boiling point. The boiling point temperature of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point temperatures of any of its constituents (a negative azeotrope).
A well known example of a positive azeotrope is 95.63% ethanol and 4.37% water (by weight).[3] Ethanol boils at 78.4°C, water boils at 100°C, but the azeotrope boils at 78.2°C, which is lower than either of its constituents.[4] Indeed 78.2°C is the minimum temperature at which any ethanol/water solution can boil at atmospheric pressure. In general, a positive azeotrope boils at a lower temperature than any other ratio of its constituents. Positive azeotropes are also called minimum boiling mixtures or pressure maximum azeotropes.
Point: MINIMUM BOILING AZEOTROPE: Constituents NOT strongly attracted to each other.
An example of a negative azeotrope is hydrochloric acid at a concentration of 20.2% and 79.8% water (by weight). Hydrogen chloride boils at −84°C and water at 100°C, but the azeotrope boils at 110°C, which is higher than either of its constituents. The maximum temperature at which any hydrochloric acid solution can boil is 110°C. In general, a negative azeotrope boils at a higher temperature than any other ratio of its constituents. Negative azeotropes are also called maximum boiling mixtures or pressure minimum azeotropes.