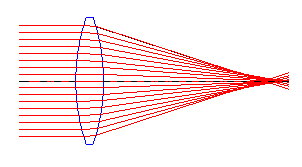
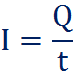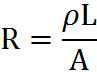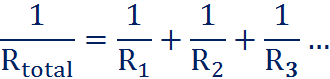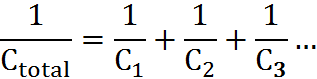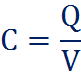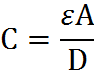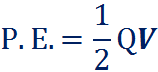 | 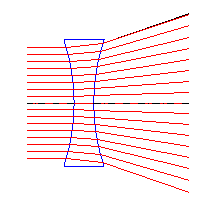 |
| Converging lens | Diverging lens |
Thursday, July 14, 2011
Converging & Diverging Lenses
Buffer Solutions
Capacitor
Wednesday, July 13, 2011
Newton's Laws
- First law: The velocity of a body remains constant unless the body is acted upon by an external force.[3][4][5]
- Second law: The acceleration a of a body is parallel and directly proportional to the net force F and inversely proportional to the mass m, i.e.,F = ma.
- Third law: The mutual forces of action and reaction between two bodies are equal, opposite and collinear.
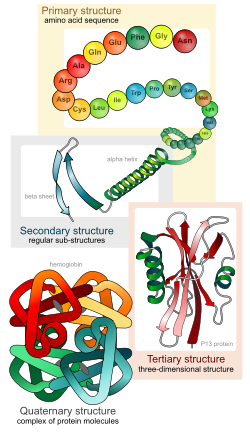
Amino Acids & Proteins
Spermatogenesis & Oogenesis (production of gametes)


Tuesday, July 12, 2011
Phase Diagram, Vapor-Liquid Equilibrium



Kaplan Pains In My Ass
Positive and negative azeotropes
Each azeotrope has a characteristic boiling point. The boiling point temperature of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point temperatures of any of its constituents (a negative azeotrope).
A well known example of a positive azeotrope is 95.63% ethanol and 4.37% water (by weight).[3] Ethanol boils at 78.4°C, water boils at 100°C, but the azeotrope boils at 78.2°C, which is lower than either of its constituents.[4] Indeed 78.2°C is the minimum temperature at which any ethanol/water solution can boil at atmospheric pressure. In general, a positive azeotrope boils at a lower temperature than any other ratio of its constituents. Positive azeotropes are also called minimum boiling mixtures or pressure maximum azeotropes.
Point: MINIMUM BOILING AZEOTROPE: Constituents NOT strongly attracted to each other.
An example of a negative azeotrope is hydrochloric acid at a concentration of 20.2% and 79.8% water (by weight). Hydrogen chloride boils at −84°C and water at 100°C, but the azeotrope boils at 110°C, which is higher than either of its constituents. The maximum temperature at which any hydrochloric acid solution can boil is 110°C. In general, a negative azeotrope boils at a higher temperature than any other ratio of its constituents. Negative azeotropes are also called maximum boiling mixtures or pressure minimum azeotropes.
Monday, July 11, 2011
Radiation, Conduction, & Convection RAP
Sunday, July 10, 2011
Mutations
Small-scale Point Mutations (one base pair)
Nonsense – premature stop codon, truncates the protein
***the mRNA sequence (with its U's) is what's mutated, thus mutating codons that code for specific proteins!!! here you've mutated a codon in the mRNA such that it now reads as a "STOP codon", which are either UAA, UAG, or UGA.
Missense – codes for a different AA
Silent – codes for the same AA (no change in phenotype)
Insertions/Deletions (from Transposons or errors)
Frameshift – shifts the reading frame
Splice site mutation – alters the splicing of mRNA
**splicing is a modification of an RNA after transcription, in which introns are removed and exons are joined!!
a genetic mutation that inserts or deletes a number of nucleotides in the specific site at which splicing of an intron takes place during the processing of precursor messenger RNA into mature messenger RNA. The abolishment of the splicing site results in one or more introns remaining in mature mRNA and may lead to the production of aberrant proteins.
Hormones (mechanisms of action)
Saturday, July 9, 2011
Colligative Properties (Quantity, not identity, matters)

ex: Adding salt to boiling water:
- actually going to take a LONGER time to boil (since the vapor pressure has been depressed, takes for heat and time to reach the solution's boiling point)
- BUT, more HOT water molecules, bound to the solute particles, will STAY in the liquid phase INSTEAD of jumping to the gas/vapor phase, so the "pasta" will COOK FASTER!
- High vapor pressure out by beach (humid atmosphere), Low vapor pressure where dry at high altitudes (Not humid atmosphere)
Evolution
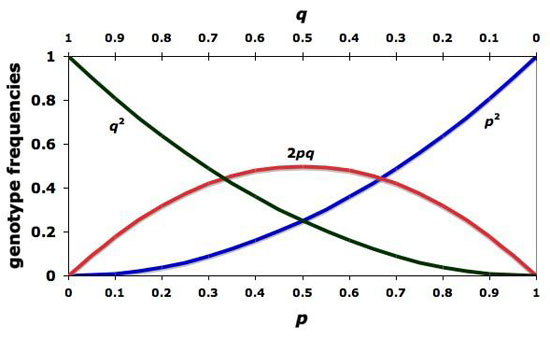
Hardy-Weinberg Equilibrium
- evolution as a result of changing gene frequencies (frequency of a particular allele) within a population
Gene pool only stable when:
1. The population is very large
2. There are no mutations that affect the gene pool
3. Mating between individuals in the population is random
4. there is no net migration of individuals into or out of the population
5. The genes in the population are all EQUALLY successful at reproducing
p2 + 2pq + q2 = 1
p + q = 1
p2 = frequency of TT (dominant homozygotes)
2pq = frequency of Tt (heterozygotes)
q2 = frequency tt (recessive homozygotes)
Cross between 2 heterozygotes:
| | p = .80 (T) | q = .20 (t) |
| p = .80 (T) | (p2 = .64) TT = 64% | (pq = .16) Tt = 16% |
| q = .20 (t) | (pq = .16) Tt = 16% | (q2 = .04) tt = 4% |
The gene frequencies of F1 generation can be calculated as follows:
64%TT = 64% T allele + 0% t allele
32% Tt = 16% T allele + 16% t allele
4% tt = 0% T allele + 4% t allele
Gene freq = 80% T allele + 20% t allele
Thus, p = .80 and q = .20. These frequencies are the same as those in the parent generation, thus demonstration H-W equilibrium in a non-evolving population. (However, this does not exist in nature!)
Directional Selection: Organisms must adapt to a changing environment, so produces an adaptive change over time which increases the proportion of individuals with an extreme phenotype (ex: DDT-resistant mosquitoes).
Stabilizing Selection: Eliminates deviations from the norm & reduces frequency of extreme phenotypes by maintaining a well-adapted population, uniform in character.
Disruptive Selection: Favors BOTH phenotypic extremes, leading to the existence of 2 or more phenotypic forms within a population (polymorphism).

Microevolution
Natural Selection: Genotypes with favorable variations are selected through natural selection, and the frequency of favorable gene increases within the gene pool.
Mutation: Gene mutations change allele frequencies in a population, shifting gene equilibria.
Assorting Mating: If mates are not randomly chosen, but rather selected according to criteria such as phenotypes and proximity, the relative genotype ratios will be affected, and will depart from the predictions of the Hardy-Weinberg equilibrium. On the average, the allele frequencies in the gene pool remain unchanged.
Genetic Drift: Genetic drift refers to changes in the composition of the gene pool due to chance. Genetic drift tends to be more pronounced in small populations, where it is sometimes called the founder effect.
Gene Flow: Migration of individuals between populations will result in a loss or gain of genes, and thus change the composition of a population’s gene pool.
Thursday, July 7, 2011
Wednesday, July 6, 2011
Monocistronic vs. Polycistronic mRNA

Tuesday, July 5, 2011
Fluid Mechanics

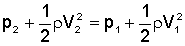
Continuity Equation: Q = A1v1 = A2v2
 Poiseuille's Principle
Poiseuille's PrincipleAn equation which describes laminar flow in a straight tube. V=P¹r4/8nl, where V= flow P= driving pressure r= radius of tube n= fluid viscosity l= length of tube.
"Thus, when one describes a patient as having "high blood pressure", it means that their heart is generating a very large P1 in order to produce the necessary Q to get blood to the tissue in a timely fashion."
http://www.youtube.com/watch?v=-jyBY726np4&feature=results_video&playnext=1&list=PL05679B8070040C79
 The Hydraulic Press
The Hydraulic Presshttp://www.youtube.com/watch?v=TjzKpke0nSU

Magnetic Force = Centripetal Force

You can change the magnetic force by changing B or v or maybe even q. You might think, then it will no longer be equal to mv^2/r. But r will change so that mv^2/r changes to match Bqv.
Imagine you increase the magnetic field. Then the charged particle will make a tighter turn. If you decrease it, it makes a wider turn. Either way, it keeps turning the same direction, and eventually comes back to where it started.
If it moves in a circle at a constant speed, then Bqv = mv^2/r must be true for some value of r.
Why does it move in a circle at a constant speed? The assumptions are that there are no other forces on the particle and that the B field is uniform. The magnetic force is always perpendicular to the direction of motion. A perpendicular force can do no work, so it can't add or remove kinetic energy. That explains the constant speed. When something moves in a circle, the direction of motion, which is tangent to the circle, is perpendicular to the acceleration, which points to the center along the radius of the circle. That explains why a circle is a path that can result from a perpendicular force."
Electrical Circuits

Current is the flow of electrons (or charge) per unit time:
Where ‘Q’ is the charge, and ‘t’ is the time in seconds.
Ohm’s Law: the voltage of a circuit is the product of the current and the resistance:
![]()
Where ‘V’ is the voltage, ‘I’ is the current, and ‘R’ is the resistance.
Resistance, Resistivity: the resistance of a metal/wire/material is the following equation:
Where ‘ρ’ is the resistivity of that substance, ‘L’ is the length of the substance (or wire, which is usually used for this type of problem), and ‘A’ is the cross-sectional area of the said wire.
As the equation shows, the longer the wire, the more resistance; the greater the cross-sectional area of the wire, the less resistance!
RESISTANCE:
Measured in ‘ohms’ (Ω), the resistance can be either in series or parallel. If in series, the path of the current can only go through that resister (there is no other path for the current to go). However, if the resisters are in series, then the current can go through any resister or wire that the current decieds to go through.
RESISTERS IN SERIES:
Rtotal = R1 + R2 + R3 ….
RESISTERS IN PARALLEL:
You must always calculate the resitance with keeping in mind whether the resisters are in series or in parallel!
Kirchoff’s Laws:
Kirchoff has two very important laws to solve for any circuit problem that may be on the MCAT:
1. The sum of all current (i) entering a junction equals the current leaving that junction. Another way to say this rule is that the sum of current in a junction equals zero.
2. The total voltage across any circuit equals zero (![]()
Using the above two laws, it is possible to solve for practically any circuit problem on the MCAT.
Anmeter: this measure current.
Votage Meter (Galvanic Meter): as this sounds, this measures voltage.
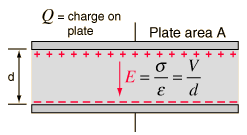
CAPACITORS:
Capacitors store charge, and release it when the orginal voltage across the circuit is stopped (such as by a battery). When the original emf is stopped, the capacitor acts as the new emf, and releases its charge across the circuit.
***The equations for capacitors are exactly the opposite than for resistors:***
CAPACITORS IN SERIES:
CAPACITORS IN PARALLEL:
Ctotal = C1 + C2 + C3
The strength of capacitors (basically, the ability for the capacitor to store charge) is dependent on a variety of factors:
1. the cross sectional area of the metal plate
2. the distance between the plates
3. the material between the plates
The ‘capacitance’ (the ability to store charge) of a capacitor is calculated as such:
Which tells you that the capacitance is the charge divided by the voltage of that charge. Meaning, the amount of charge flowing in directly proportional to the capacitance, while the voltage drop across the plates is inversly proportional to the capacitance. This is because if the voltage across increases, the less the capacitor would be able to store before it has to release the built-up charge.
![]()
This tells you that the voltage is the product of the electric field strength and the distance between the plates. As you increase either one, you increase the voltage (which, according to the equation before) thereby decreasing the capacitance.
The equation above tells you that the capacitance of a material is porportional to both the ‘dielectric’ (the ability of the inside material to insulate the plates, thereby increasing the ability for the capacitor to store the charge before releasing it) and the area of the plates. However, it is inversly porportional to the distance (as we saw in the equation before as well).
The potential energy of a capacitor can be calculated in the following equation:
Where ‘Q’ is the charge buildup and ‘V’ is the voltage potential. With the above equation, it is possible to move things around by substituting ‘Q’ with ‘CV’ and so forth.
Source: http://phasing.org/2010/03/14/electrical-circuits/