Thursday, June 30, 2011
Wednesday, June 29, 2011
Internal Energy (1st Law of Thermodynamics) & Sign Rules
q (+) = heat absorbed (energy is added to the system)
q (—) = heat evolved (energy is subtracted from the system)
W (+) = work done on the system (energy is added to the system)
W (—) = work done by the system (energy is subtracted from the system)

Tuesday, June 28, 2011
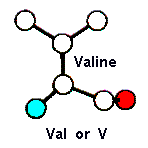
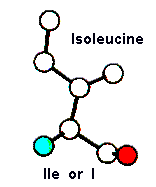
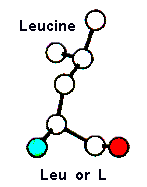
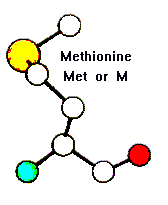
Amino Acid TERMS
- The genetic code is composed of nucleotide triplets. In other words, three nucleotides in mRNA (a codon) specify one amino acid in a protein.
- The code is non-overlapping. This means that successive triplets are read in order. Each nucleotide is part of only one triplet codon.
- The genetic code is unambiguous. Each codon specifies a particular amino acid, and only one amino acid. In other words, the codon ACG codes for the amino acid threonine, and only threonine.
- The genetic code is degenerate (redundant!). In contrast, each amino acid can be specified by more than one codon.
- The code is nearly universal. Almost all organisms in nature (from bacteria to humans) use exactly the same genetic code. The rare exceptions include some changes in the code in mitochondria, and in a few protozoan species.
Monday, June 27, 2011
Hemoglobin and the Oxygen-Dissociation Curve (IB)

- When CO2 is produced it diffuses into RBC, where it reacts with H2O and is converted into Carbonic Acid via carbonic anhydrase enzyme, ****the acid then dissociates into H+ and HCO3-
- The H+ ions made the blood more acidic which causes oxyhemoglobin to dissociate and release O2
- The HCO3- ions are pumped through the membrane of the RBC and into the plasma where they combine with Na+ to form NaHCO3
- To ensure that the RBCs remain uncharged, Cl- ions pass into them. This is known as the chloride shift!
- When the RBCs reach the lungs, the NaHCO3 combines with H+ to form H2O, CO2, and Na+
- The CO2 is then released from the body during exhalation
Partial Pressure of CO2
- As cells respire, the pO2 decreases, and the pCO2 increases
- An increase in the pCO2 causes Oxyhemoglobin to give up its O2 more readily
- This is because when the CO2 is converted to acid it produces H+ ions which lowers the pH of the blood
- The Oxyhemoglobin then dissociates, giving up its O2 so that the Hb can bind to the H+ ions to prevent a change in pH
- Therefore Hb acts as a buffer by taking up the H+ ions and forming "Hemoglobinic Acid"
- more oxygen released with increase in CO2 and lowering of pH levels
- as pCO2 increases, the dissociation curve shifts to the right & down
- When O2 diffuses into the lungs there is a high PP of O2, so it will load onto Hb forming Oxyhemoglobin
- The O2 is then carried in the blood to respiring cells that have a low pO2 and a high pCO2
- So the Oxyhemoglobin releases the O2 because of the low pO2 and ALSO because of the high pCO2 which causes a DROP in pH
- The O2 is used by cells for respiration and the Hb binds to the H+ ions to prevent changes in pH
The composition and O2 carrying capacity differs among organisms...
Leslie: http://www.interactive-biology.com/2643/061-the-bohr-effect/
Transport of Respiratory Gases: http://www.youtube.com/watch?v=Qrvrs6RXxwY
Biochem dude: http://www.youtube.com/watch?v=DgelvyH7iB8&feature=related
Friday, June 17, 2011
Transmembrane Domains
Blue, Pink, (some) Green FLASHCARDS!
Ochem trix
Oxidation = Addition of O atoms (C-O bonds) / Removal of H+ atoms
ex: alkene → alcohol (2nd half of hydroboration reaction)
Reduction = Removal of O atoms (C-O bonds) / Addition of H+ atoms
ex: ketone → 2˚ alcohol
Thursday, June 16, 2011
VSEPR (Molecular Geometry)


Wednesday, June 15, 2011
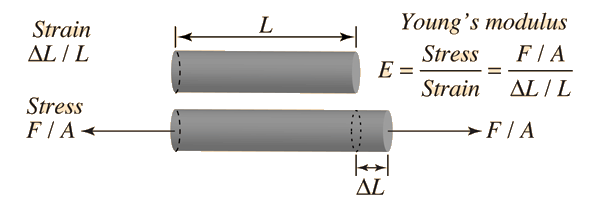
Young's Modulus Equation (Stress/Strain)
Complex Genotype Probabilities
2 complex, unlinked genes…calculate each individual probability and then calc. the PRODUCT of the probabilities!
Osmolality (blood)
Osmolality…
increases with dehydration (more concentrated)
&
decreases with overhydration (less concentrated)
Tuesday, June 14, 2011
Ascending order of blood pressure in the given vascular tissues


Hydrostatic pressure is generated by the systolic force of the heart. It pushes water out of the capillaries.
The water potential is created due to the ability of small solutes to pass through the walls of capillaries. This buildup of solutes induces osmosis. The water passes from a high concentration (of water) outside of the vessels to a low concentration inside of the vessels, in an attempt to reach an equilibrium. The osmotic pressure drives water back into the vessels. Because the blood in the capillaries is constantly flowing, equilibrium is never reached.
The balance between the two forces differs at different points on the capillaries. At the ARTERIAL END of a vessel, the hydrostatic pressure is greater than the osmotic pressure, so the net movement (see net flux) favors water and other solutes being passed into the tissue fluid. At the VENOUS END, the osmotic pressure is greater, so the net movement favors substances being passed back into the capillary. This difference is created by the direction of the flow of blood and the imbalance in solutes created by the net movement of water favoring the tissue fluid.
Continuity Equations
A x v = constant
Ionization
Chemistry Randos

Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond is also a shorter bond, however, there are also few exceptions (Ex, H-H and H-O, the latter one has longer and stronger bond).
SPECIFIC HEAT (Heat Capacity, C)
***"the amount of energy required to raise 1g of that substance by 1 degree Celsius"
- larger the value, harder it is to raise the temp (ex: wood's 2.1 > mercury's 0.1)
Log rules...ugggggh
Monday, June 13, 2011
Extreme Physics...
Lowest Freezing Point (makes no sense but remember)
The solution with the HIGHEST concentration of particles (CaCl2, NOT NaCl) will have the LOWEST freezing point.
Higher Ksp means MORE soluble…
Saturday, June 11, 2011
Vacuum
Transduction/Transfection
Friday, June 10, 2011
Electron Configuration & Decay



- Decreases the number of neutrons AND protons in large nucleus
Beta decay: Neutron decomposes into a Proton (and Electron), or vice versa via weak nuclear force
http://www.youtube.com/watch?v=1j-eDPLSBm0
*Mass number stays the same!!
- β- à TOO MANY neutrons
o Increases the number of protons
- ex: Carbon 12 vs. Carbon 14... Carbon 14 gains a P+, releases an E-
- β+ à TOO FEW neutrons
o Decreases the number of protons
Electron Capture: Converts protons into neutrons
- nucleus draws in inner-shell electron, P and E react to form a Neutron
*Mass number stays the same, only Atomic number changes!! (ex: Rb to Kr)
- Decreases number of protons, porque hay demasiado!
Gamma decay: Simply the expulsion of energy. Does not change the identity of the nucleus. Gamma photons emitted so atom can relax to its ground state.
- Gamma rays are emitted by the nucleus! and are often used in medicine to KILL CELLS
http://www.mcatquestion.com/findquestion.php?arg1=1044
one of like a trillion HALF-LIFE EQUATIONS:
Thursday, June 9, 2011
Gradients (all about the SOLUTE)
The plasma membrane of a cell lets water pass freely. Water will flow toward the side that has the most dissolved molecules (called solutes). Said another way, water will flow from a hypotonic solution toward a hypertonic solution until both sides of the membrane reach equilibrium.
- a HYPERTONIC solution has more solutes than cell cytosol (H2O flows OUT of cell)
- - cytoplasm of the cell has a lower solute concentration than the extracellular medium
- a HYPOTONIC solution has less solutes than cell cytosol (H2O flows INTO the cell)
- - cytoplasm of the cell has a higher solute concentration than the extracellular medium
- - too much inflow, cell my lyse
- - “Kaplan Exclusive!”: Look how the O in hypotonic looks like a SWOLLEN cell!
- an ISOTONIC solution has the same solute concentration on both sides.

- If a cell is in a Hypertonic solution, the cell will shrink.
If a cell is in a Hypotonic solution, the cell will expand.
If the cell is in an Isotonic solution, the cell will remain the same.
If the cell is Hypertonic with respect to the medium, it will expand, this infers that the solution it is in, the medium, is Hypotonic.
If the cell is Hypotonic with respect to the medium, it will contract(shrink), this infers that the solution it is in, the medium, is Hypertonic.
Good student-Doc thread on urine hypertonicity relative to blood: http://forums.studentdoctor.net/archive/index.php/t-833556.html
Stoichiometry trix
Limiting reagent: NOT nec. species in smallest amount, but one that is CONSUMED FIRST
***pay attention to consumption behavior, not initial quantities!
grams1 à moles à [stoich REAC/PROD] à grams2
1) BALANCE the equation!!!
2) Do flow chart with ONE of the products--doesn't matter which, just pick one and STICK to it for the sake of comparison!!
3) Less gram #2 value is LR
http://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
****calculate number of MOLES (without stoich. numbers) first...
FONClBrISCH (most electroneg to least electroneg)
- sum of oxidation numbers must equal overall charge (indicates what the atom is doing with its valence electrons when it forms a compound)
Wednesday, June 8, 2011
Electrochemistry
- Eo is a type of energy per electron so it remains unchanged even if we double the numbers of reactants and products in the reaction
- If an equation is reversed (so that the reactants become the products), the sign of Eo is also reversed
- The cell's emf (electromotive force, often referred to as the cell voltage), is calculated by adding together the Eo values for each half reaction:
Eocell = Eoreduction + Eooxidation - The reaction is spontaneous in the direction as written if
Eocell > 0
(Eocell positive) - The reaction is spontaneous in the reverse direction to that written if
Eocell < 0
(Eocell negative) - A galvanic cell (voltaic cell) produces electricity so the overall cell reaction must have a positive Eocell value
(Eocell > 0) - http://www.ausetute.com.au/calcelemf.html
Ecell must be > than 0 for the reaction to be spontaneous! So by knowing how to subtract what, you know WHICH “half-cell” is the cathode, and which is the anode.
*If both half-reactions appear to be reductions, the OPPOSITE direction of each is oxidation.
Electron movement (wire)
***ELECTRONS ALWAYS MOVE "AWAY" from ANODE, and "COME TO" the CATHODE.
CATHODE = site of REDUCTION
ION movement (salt bridge)
ANIONS (-) move to the ANODE
CATIONS (+) move to the CATHODE
***ANODE and CATHODE are just sites/locations in space (ex: California & New York), determined by the movement of IONS...do not confuse!!!
|
| Electrochemical Cells | |
Oxidation-reduction or redox reactions take place in electrochemical cells. There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; nonspontaneous reactions occur in electrolytic cells. Both types of cells containelectrodes where the oxidation and reduction reactions occur. Oxidation occurs at the electrode termed the anode and reduction occurs at the electrode called thecathode.
Electrodes & Charge
The anode of an electrolytic cell is positive (cathode is negative), since the anode attracts anions from the solution. However, the anode of a galvanic cell is negatively charged, since the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The cathode of a galvanic cell is its positive terminal. In both galvanic and electrolytic cells, oxidation takes place at the anode and electrons flow from the anode to the cathode.
Galvanic or Voltaic Cells
The redox reaction in a galvanic cell is a spontaneous reaction. For this reason, galvanic cells are commonly used as batteries. Galvanic cell reactions supply energy which is used to perform work. The energy is harnessed by situating the oxidation and reduction reactions in separate containers, joined by an apparatus that allows electrons to flow. A common galvanic cell is the Daniell cell, shown below.

Electrolytic Cells
The redox reaction in an electrolytic cell is nonspontaneous. Electrical energy is required to induce the electrolysis reaction. An example of an electrolytic cell is shown below, in which molten NaCl is electrolyzed to form liquid sodium and chlorine gas. The sodium ions migrate toward the cathode, where they are reduced to sodium metal. Similarly, chloride ions migrate to the anode and are oxided to form chlorine gas. This type of cell is used to produce sodium and chlorine. The chlorine gas can be collected surrounding the cell. The sodium metal is less dense than the molten salt and is removed as it floats to the top of the reaction container.