
DO:
1) Lower the Ea of the reaction
2) Increase the RATE of the reaction
DO NOT:
3) Do not affect the ΔG (free energy)
- energy from initial to final state
4) Are neither changed nor consumed during a reaction
5) Do not alter the equilibrium constant (K)!!!
Enzymes vary in their specificity to a substrate and are specific for a particular reaction or class of reactions. The most promising theory that accounts for ES complex formation is the "Induced Fit Hypothesis" in which the active site conforms to the contour of the substrate instead of maintaining a definitive shape ("Lock & Key Theory").
- Cookie monster eats chocolate chip cookies, not oreos
Enzymes may require co-enzymes (non-protein molecules) to be catalytically active. These may either help with substrate binding or induce the ACTIVE, as opposed to the alternate inactive, allosteric conformation.
- Cookie monster's allostery means he's either got on inhibitory Orthodontic headgear or is open-mouthed and cravin' some kooks
Enzyme affinity can be read from a chart that plots "reaction velocity (V)" and "substrate concentration (S)" against each other. Vmax is the tapering off of the reaction curve that says the enzymes are full and cannot increase the rate of reaction anymore. 1/2 Vmax is at the midway point of the vertical curve that says exactly half of the binding sites are open--the most ideal state for operating enzymes. Substrate concentration Km will correspond to 1/2 Vmax on the x-axis. A low Km means ES affinity is HIGH (if the reaction req. a short period of time, the affinity must be strong!). A high Km means the affinity is LOW (the ideal rate is taking a long time).
- Cookie monster has reached Vmax when his mouth is so full of cookies he can no longer fit anymore. He is at 1/2 Vmax when he's been offered a certain number of cookies (Km) at a comfortable pace such that he can swallow them in a generally useful fashion. A low Km (preferred) means he's been starvin' marvin'. A high Km means he had Applebee's curb-side to go before cookie time.
The Km is a little more confusing only because some people mix up a large value with a small value. For example, compare 1x10^-10 M to 1x10^-3 M. The 1x10^-3 M is much larger than 1x10^-10 M, and the larger the Km the lower the affinity of the substrate for the enzyme. Thus a high Km means it takes a greater concentration of substrate for the enzyme to be half saturated compared to a low Km which means it only takes a very little bit or a very low concentration of substrate for the enzyme to be half saturated (half maximal velocity)."
Enzymes are sensitive to pH and temperature conditions. Ideal pH is 7.4 (with stomach and pancreatic exceptions) and temperature is 40°C. Rates of reactions tend to double with 10°C increments.
Enzymes can also be inhibited by 1) Feedback inhibition--later product in cascade inhibits earlier enzyme in cascade, 2) Competitive Inhibition--compete with substrate's active site, or 3) Noncompetitive Inhibition--forms covalent bond with enzyme.

Allosteric enzymes
Binding of effectors to regulatory subunits
Binding of effectors to regulatory subunits
Allosteric enzymes may also have regulatory subunits that bind either activators or inhibitors. Activators and inhibitors are termed "effectors." Inhibitors cause the allosteric enzyme to adopt the inactive shape. Activators promote the active shape.
An equilibrium exists between the active and inactive shapes. The amount of active and inactive enzyme is dependent on the relative concentrations of substrate and inhibitor, as suggested by the diagram:
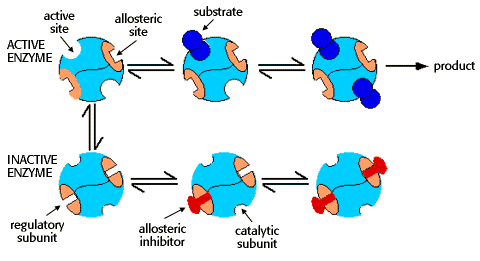
The binding of an allosteric inhibitor causes the enzyme to adopt the inactive conformation, and promotes the cooperative binding of a second inhibitor.
An excess of substrate can overcome the inhibitor effect. Substrate binding causes the enzyme to assume the active conformation, and promotes the cooperative binding of additional substrate, leading to product formation."
The meaning of the S-shaped Curves with and without inhibitor
In the presence of inhibitor (plus inhibitor), higher concentration of substrate is required to shift the enzyme to the active conformation. However once a high enough concentration of substrate is reached to promote active shape, the substrate binds cooperatively (S-shaped curve), and the same maximum rate is achieved as without inhibitor.

Source:
No comments:
Post a Comment