
Water is not included in the acid-dissociation equilibrium expression because the [H2O] has no effect on the equilibrium.
As the Ka value of an acid increases, so does the strength of the acid. By definition:
- strong acid: Ka > 1
- weak acid: Ka < 1
The larger the value of pKa, the smaller the extent of dissociation. A weak acid has a pKa value in the approximate range −2 to 12 in water. Acids with a pKa value of less than about −2 are said to be strong acids; a strong acid is almost completely dissociated in aqueous solution, to the extent that the concentration of the undissociated acid becomes undetectable.
Larger the Ka, stronger the acid!
Smaller the pKa, stronger the acid! (THE REVERSE)
pKa + pKb = 14
pH + pOH = 14
pH = -log[H+] à [H+] = 10-pH
pOH = -log[OH-] à [OH-] = 10-pOH
Henderson-Hasselbalch:
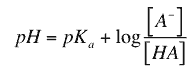
Equivalence point: amount of ACID EQUAL to amount of BASE present, only ions exist in solution
1/2 Equivalence point: (pH = pKa) volume added is half of what it will be at equivalence point & the protonated and deprotonated states are equal
| Type of Titration | When Equivalence Point will occur |
| Weak Acid w/ STRONG BASE | pH > 7 |
| Weak Base w/ STRONG ACID | pH < 7 |
| STRONG ACID w/ STRONG BASE | pH = 7 |
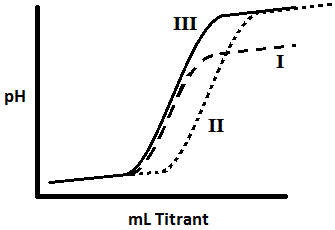
The graph above is a titration curve of three different solutions. Solution I is titrated with a base of a lower pH. Rank the solutions in terms of the strength of the acid in the solution.
(a) I < II < III
(b) III < I < II
(c) II < III < I
(d) There is no difference among the strength of the acids.
Explanation: Consider what a titration involves and what this curve tells us about the solutions involved. In this titration we are starting with an acid solution (low pH before titrant is added) and are titrating with a base (high pH after titrant is added). After adding a certain amount, the proportion of acid/base begins to approach 1/1 and the pH will start to increase. As we continue adding base, the pH will eventually level off as the ratio of base far exceeds the ratio of acid.
Using a titrant of lower pH would result in graph I, since the starting point is the same but the end point is lower. Using a higher concentration of acid would result in graph II (compared to graph III), because it takes more titrant to reach the same end point of the titration (the start and end pH values are the same, but the amount of base required is greater; therefore the concentration of starting acid is greater). Thus we can conclude that there is no difference in the strength of the acids; we are looking at three different experimental conditions.
Using a titrant of lower pH would result in graph I, since the starting point is the same but the end point is lower. Using a higher concentration of acid would result in graph II (compared to graph III), because it takes more titrant to reach the same end point of the titration (the start and end pH values are the same, but the amount of base required is greater; therefore the concentration of starting acid is greater). Thus we can conclude that there is no difference in the strength of the acids; we are looking at three different experimental conditions.
***Compare pH and pKa's quickly:
- Recall that when the pH is below the pKa of a titratable group, the group will be predominantly protonated
- More detail: Basic groups, like amino groups, have high pKas, whereas acidic groups like carboxylic acids have low pKas (recall that Ka = [A-][H+]/[HA]; therefore an acidic group which favors dissociation ([A-][H+]) will have a higher Ka. The pKa is the -logKa, so a higher Ka will result in a lower pKa (do the math to compare the pKas of high and low Ka values).

No comments:
Post a Comment